What is Steam Cracking?
by: Souad LOUSDAD
Alongside with the “Conversion Processes “and the “thermal cracking”, there is the “steam cracking” or “Pyrolysis”.
Hydrocarbon steam cracking is one of the most important processes in the petrochemical industry, as it is able to produce highly valuable olefins such as ethylene, propylene and butadiene from lower value feedstocks, which usually have fossil fuel origin and range from gaseous feedstocks like ethane and propane, to liquid, heavier feedstocks, such as naphtha, gas oil and gas condensates. From the mentioned feedstocks, naphtha is the most widely used, due to availability, low cost and potential for producing yields of olefins, namely ethylene and propylene.
It is exclusively based on thermal cracking, which occurs in the presence of steam, breaking down the molecule by higher temperature.
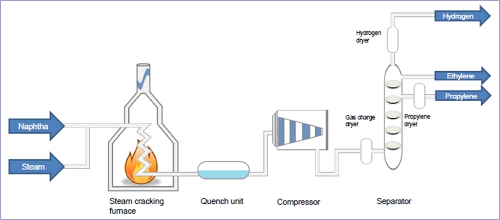
First, the steam cracking unit is divided to three main sections:
- The hot section: it includes the furnace, the primary fractionator and the quench.
- The compression section: it involves the compressors, the caustic scrubbers and the dryers.
- The cold section: it is the part of the unit where the Pyrolysis products are separated; it includes the demethanizer, the de-ethanizer, the depropanizer and the debutanizer…
The cracking furnace is the “heart” of any steam cracking plant; it plays the major role of a chemical reactor.
It generally includes two sections, according to the type of heat transfer: the convection section and the radiant section.
Now, how does it work?
Low pressure, slightly above the atmospheric should be maintained.
The naphtha feedstock enters the furnace from the upper part to the convection section, the water vapor right below it to the same section, both pre-heated separately to the temperature of 150-600°C, after that the mixture finds its way to the radiation section where the temperature goes up to 800-850°C.
The effluents drawn off from the furnace are then cooled faster in the quench boiler to the temperature of 190-200°C in order to avoid that the temperature of 800-850 °C reached earlier in the radiation section of the furnace would change its composition by the formation of heavy polymerization products and the increase of the gasoline content, in other words it stops the undesirable reactions that prevent the formation of coke.
It is highly important for the transfer line between the furnace and the quench boiler to be as short as possible to avoid additional residence of effluents at elevated temperature, meaning to avoid a longer time of residence.
The short time of residence reveals of the high speed of heat transfer.
After that, the effluents enter the primary fractionation column, kind of a gasoline fractionator, where they are separated:
The heavy residue from the bottom is sent back on to the fractionation, the gasoline is drawn off as a side stream, and the diluent steam is condensed at multiple levels and purified from re-using while the light gaseous products from the top of the column (Pyrogase) are sent to the compression section, to be separated later.
The effluents are compressed in multiple stages (4 or 5) with intermediate cooling to prevent any heating that could lead to undesirable polymerizations.
Before the last step of compression, the gases are desulfurized by caustic scrubbing to eliminate the sulfur content in the form of H2S, COS and light mercaptans as well as CO2, and finally the gases are dried (< 1ppm) to prevent the formation of ice crystals during the process of cooling.
At the end of the compression section, we have our liquid/gas phase fractions ready to enter the next section: The cold section.
In the cold section, the effluents are sent to a demethanizer where methane (CH4) is condensed at the top along with pure hydrogen (H2) while the bottom product is sent to another column, de-ethanizer that aim to separate the C2 fraction (ethane + ethylene) at the top.
The C2 fraction from the top of the de-ethanizer emerges after in an ethane-ethylene separator where ethylene is drawn off from the top while ethane from the bottom.
The gas mixture from the bottom of the de-ethanizer is sent a depropaniser where the C3 fraction is separated, leaving from the top to a propane-propylene separator where propylene leaves from the top and propane from the bottom. The remaining from the bottom of the depropaniser is then sent to a debutanizer where the C4 mixture is recovered as the top product while the heaviest C5+ are recovered as the Pyrolysis gasoline/ bottom product.
This section definitely runs intermediate treatments to purify ethylene and propylene from alkynes, mostly acetylene and methylacetylene via selective hydrogenation.
Despite all the precautions taken to minimize coke deposits on the tubes of the furnaces and the quench boilers, it is impossible to 100% eliminate it.
Furnace operation must then be interrupted periodically to remove the coke: that is what we refer to as decoking; two successive decoking operations are usually run on a period called run-length.
As an example, for a steam cracker producing 200000t/year of ethylene in five furnaces: One is always pursuing decoking and the four others carry the production.
The operation of decoking starts slowly with injecting low air contents, like 1% and then this amount goes progressively to 15% to better control the exothermicity of the coke combustion. Another way to eliminate coke involves using the water vapor at very high temperatures 900-950°C but it could take approximately 30 hours, adding 10% of air decreases it to 10 hours, adding 20% takes the operation to only 5 hours: It is all to say that the best way is to involve both air and water vapor.
At the end, the steam cracking provides principally ethylene, then propylene, butadiene and the gasoline fraction rich with aromatics.
Now let’s sum it all up:
The steam cracking process or Pyrolysis is one of the conversion processes that depend on high temperatures to crack molecules into smaller and more valuable ones, in the first place ethylene: as the temperature goes higher, the yields of ethylene increases.
The steam adds a huge value to the process as it lowers the pressure of the whole operation and the partial pressure of hydrocarbons during the reaction of cracking, another way to boost the formation of light olefins and to avoid the composition of coke.
The residence time is one of the conditions that should be taken seriously as much as the temperature especially in the furnace: The shortest the time, the greatest the ethylene yields.
The feedstocks could be gaseous such as ethane and propane as it could be liquid like naphtha and gas condensates, that is why the conditions could change and eventually the products yields also differ.