what is Scale?
Barium and strontium sulfates form in wells that produce waters naturally saturated with barium or strontium sulfates, or when produced water containing barium or strontium is contaminated with a sulfate-bearing water. When seawater injection is employed, the seawater contains sulfate, and the
formation water may contain barium and/or strontium which will then form barium and/or strontium sulfate when the two waters commingle. The solubility of barium sulfate is very low, which means that it will precipitate even at very low concentrations.
Scale Inhibitors
Normally, two types of scale-inhibitor chemistry are used: phosphonates and/or polymers. These products are water soluble, and have a pH in the range of 2 to 7. The “scale squeeze” is an operation where long-term protection against mineral scale precipitation from the perforations is provided through the topside process. Due to injection-water breakthrough or a high content of calcium and bicarbonate in the formation water, increased scale formation may be experienced at some point in the operation of a field. In the scale squeeze, a calculated volume of scale inhibitor is displaced directly into the formation. The scale inhibitor adsorbs to the formation surface and then de-adsorbs as it returns, dissolved in the produced water. This will protect the perforating zone, the production tubing and the topside against scale precipitation. The lifetime for a scale squeeze is normally 180-365 days, but it depends on the water production rate.
Scale inhibitors prevent scale deposits in well tubulars and field flow lines to help maximize production volume and reduce downtime. Advanced formulations modify crystal growth using functionalized polymers and other chemistries to slow and prevent scale crystal formation.
The inhibitors act in a threshold process that requires only a small number of growth-inhibitor molecules. In contrast to inefficient, costly chelation methods (such as those using EDTA – ethylene diamine tetraacetic acid), chemical volumes are reduced for improved treatment economics.
In most applications, low dosage rates of concentrated formulations effectively treat the scaling problem. In severe scaling conditions, particularly with BaSO4, dose rates can be easily increased as required by formulation activity and inhibitor-to-scale specificity.
Scale inhibitors are based on three types of compounds: 1) phosphate esters, 2) phosphonates and 3) polymers.
Phosphate Esters
Phosphate esters are more tolerant of acid conditions than polyphosphates. Stable to temperatures of 150°F-160°F (65-71°C), they can withstand temperatures of 180°F (82°C) – 200°F (93°C) for a few hours.
Within these temperature limitations, phosphate esters are generally very effective calcium carbonate (CaCO3) and calcium sulfate (CaSO4) inhibitors. Except in acid environments (pH < 5.5), they also provide excellent control of strontium sulfate (SrSO4) and barium sulfate (BaSO4) precipitation. In general, phosphate esters are soluble in and compatible with high-calcium brines.
Phosphonates
Several different types of phosphonates are used as scale inhibitors. Each has different characteristics of thermal stability, calcium tolerance and efficiency relative to scale type. A broad range of characteristics are available because phosphonate scale inhibitors are supplied in acid form or with any portion of the acidity neutralized by ammonia, amines or alkaline hydroxides.
Organic Polymers
Organic polymers are chiefly crystal distorters although they also reduce precipitation in typical oilfield brines. By modifying or distorting crystal shapes, organic polymers (primarily low molecular weight polyacrylics) prevent scales from growing and adhering to equipment surfaces. Stable to 400°F (204°C) or higher, polymers are generally effective at very low concentrations for control of CaCO and BaSO4 in waters containing low concentrations of scale-forming ions. They are also effective under acidic conditions, particularly in the control of BaSO4. Polymers are often blended with other types of scale inhibitors to obtain a single product with a broader range of applications.
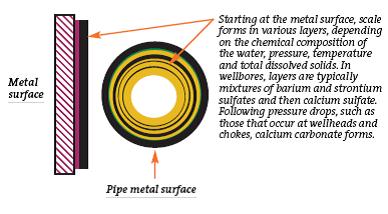
Scale Deposits
Scale is often defined as deposits of insoluble inorganic minerals. Common oilfield scales include calcium carbonate, barium sulfate, and metal sulfides.
While calcium carbonate scales deposition depends partially on pH and pressure, scale deposits generally occur when waters from different sources with different ion contents are mixed. The resulting deposits can quickly block tubulars and stop production. In many cases, scale deposits can be dissolved, but for some scales (calcium fluoride in particular) mechanical scale removal is the only remedy.
Scale Control Support
Scale prevention can occur at several levels in the process. The control method or methods are often based on the application methods available as well as the economics of the process. Engineering and analytical support is the first step in determining whether a scale problem requires an inhibitor or a remover. A review of operating conditions and possibly laboratory testing also allows selection of the most appropriate formulation.